

If you are looking to buy kratom in the United States and have been overwhelmed by the sheer amount of kratom vendors out there, you are not alone. Whether you are buying kratom from online kratom vendors or physical stores, due diligence is required to pick the best kratom vendor in the market. In this blog, we discuss the essentials of buying high-quality kratom that prioritizes consumer safety and quality.
There are many avenues for purchasing kratom available, some more convenient than others, as well as community-like settings for an intimate kratom experience.
The most convenient place for buying kratom is online. Online kratom vendors offer all popular kratom strains and products. Online kratom products are also easily browsable on the web and you can make price comparisons between your selected kratom brands.
Another advantage of buying kratom online is that you can verify if the kratom vendor is credible, GMP-approved, and well-reputed. You can read customer reviews, learn more about the vendor’s manufacturing practices, and lab-testing procedures and ask any questions via email.
Since 2016, online kratom vendor Super Speciosa has been perfecting ways to bring you kratom as nature intended – unaltered, untouched, and uncompromised.
Kratom products are also available in physical stores such as kratom shops, health food stores, specialty smoke shops, and even gas stations.

If you have always been curious to get a closer look at the variety of kratom products, you can search for nearby kratom stores and pay a visit. Shopping in-store offers a personable kratom buying experience and you can always clear up any concerns with the support staff right away.
In-store staff are also knowledgeable about kratom and can guide you about the best practices of using kratom supplements and how you can add kratom to your wellness regimen. However, brick-and-mortar kratom stores are relatively pricier than online kratom vendors.
Kratom conventions and special events are especially exciting for the kratom industry and community as it offers networking and engagement opportunities with kratom manufacturers and users all over the country. You can always look up upcoming kratom expos and trade shows near you such as the Las Vegas Kratom Expo and White Label World Expo.
These trade shows are held in different locations throughout the year where different businesses like kratom, tobacco, vape, and CBD companies come together and showcase their products for sale or wholesale businesses.

Another exciting and growing social avenue for the kratom community are kava and kratom bars. If you have been searching for an enjoyable bar-like experience with kratom beverages prepared by professionals, kratom bars are a great option.
Kava and kratom bars are like cafes/bars but they don’t serve alcohol. Instead, these kratom bars serve different herbal drinks and tonics made from kava or kratom. Kava bars are great sober alternatives to enjoy your evenings without the hangovers of alcoholic beverages.
Most people hang out at kava bars to socialize and chill out in the evening to relax. You may find some packaged kratom products to buy, but most kava bars offer exotic kratom beverages blended with a variety of kratom products.
When buying high-quality kratom, you need to know what you are looking for whether that’s quality, vendor reputation, or different strains.
Verifying quality should be at the top of your checklist when buying kratom. Credible kratom vendors are transparent about how they source kratom and manufacture kratom products.
Most of the kratom that comes to the U.S. is from the West Kalimantan region of Indonesia. However, there is a lack of quality control from the supplier side which is why 15 to 25% of all kratom fails microbial testing standards.
Kratom is a natural product exposed to all kinds of elements and some processors even mill the leaves in open facilities which can increase the risks of contamination.
At Super Speciosa, we test all our kratom with the assumption that we do not know who the supplier is. This has led us to design manufacturing facilities to ensure maximum consumer safety.
Another essential is to buy kratom from reputable vendors who have been in the market for some time. As new kratom stores pop up every week, not all of them prioritize consumer safety and follow good manufacturing practices. A credible vendor will have an established reputation and customer base.
There are multiple Reddit groups like r/Vendorsofkratom, that share customer experiences with different vendors. You can also check out Kratom Support Facebook groups, like Super Leaf Warriors to help find the right kratom products for you.
Kratom varieties and strains offer diverse kratom experiences but a kratom store with lots of strains is not a good sign. In fact, the usage of “strain terminology” is a misnomer as no harvester on the supply side is cultivating different strains or colors based on their unique properties.

The strain names are arbitrarily made up by the vendor. For example, the “Red Thai” strain is not from Thailand, as 99% of the kratom in the United States comes from Indonesia. The colors also do not reflect the vein colors of the processed leaves as multiple colored veins are found on the same tree. The color, instead, comes from different drying and harvesting techniques. Kratom that is left to dry outdoors and in the sun for longer periods of time has a darker green hue, which is known as the “red vein strain”. Kratom that is dried indoors and has a brighter green hue is known as green vein and white vein kratom.
At Super Speciosa, we like to rely on the term “variety” instead of “strain”. We separate and grade kratom based on batches and alkaloid profiles. Our Maeng Da and Signature Reserve are the strongest in Mitragynine percentage followed by the milder Bali, Malay, and Thai strains.
This provides our customers with more consistency and a clearer understanding of what they are purchasing. For more information, read the truth about kratom strains.

When buying kratom, one non-negotiable is whether the company is certified by the American Kratom Association.
AKA’s GMP Standards Program requires every approved kratom vendor to have successfully demonstrated that their kratom products are safe, high-quality, and adequately labeled for consumers to make an informed purchasing decision.
Kratom vendors like Super Speciosa that maintain the high standards of this program are listed as an AKA Qualified Vendor and their products bear the “AKA GMP Qualified” seal on their packaging.
When kratom companies are certified by the AKA, it gives kratom users peace of mind knowing that their kratom is safe to consume.
No certification is a big red flag. This means the kratom vendor can be packaging kratom straight from their supplier without any quality checks for contamination. These companies are known as “Garage Vendors” and should be avoided.
It’s always beneficial to check out reviews from real customers on the kratom company and its products. At Super Speciosa, all our product pages have hundreds of customer reviews by our verified kratom buyers that give you authentic anecdotal accounts of our kratom supplements.
Another important factor to look out for when buying kratom is to make sure the vendor has third-party lab testing from an accredited lab for each batch of every product. The lab test results should show alkaloid levels, as well as show the product is free of harmful levels of microbials, contaminants, and heavy metals.

Super Speciosa’s kratom products come with an easily scannable QR code that shows the lab-test certificate of the exact batch. Some red flags to look out for are no traceable batch numbers, fake certificates without proper lab information, or testing only for a few contaminants. Read more about Super Speciosa’s lab testing.
Be cautious of brands making any therapeutic or medical claims on their kratom product. Claims cannot be made for labeling and marketing purposes, including on their website, blog posts, social media, advertisements, and even reviews (that are controlled by the vendor).
Claims can be misleading, as kratom’s effects can vary from person to person. There are also other factors such as serving size, tolerance level, brain chemistry, physiology, etc.
Avoid gas station kratom as you may not be able to verify the credibility of the kratom products or whether the kratom is lab-tested and follows good manufacturing practices.
Apart from the AKA GMP approval seal, here are a few things to look for on the label of kratom products.
Ask the kratom vendor about their manufacturing and lab-testing processes. Some questions you can ask are:
Kratom is not legal everywhere in the United States of America. In some states, cities, and counties, it is a controlled or scheduled substance. Super Speciosa doesn’t ship to any address where kratom is illegal.
States where kratom is illegal: Alabama, Arkansas, Indiana, Rhode Island, Vermont, and Wisconsin.
Cities and counties where kratom is currently illegal: Sarasota County, FL, Union County, MS, San Diego, CA, Jerseyville, IL, Oceanside, CA, and Ontario, OR.
Visit ProtectKratom.org to learn more about kratom legislation or support the American Kratom Association actively working to protect access to kratom.
In 2016, our founders, Ken and Aaron, started Super Speciosa with a vision of ‘kratom with a conscience’, committed to providing high-quality, safe, and clean kratom products. Super Speciosa built a quality system from scratch, tailored specifically for kratom, to ensure the delivery of the best possible product to their customers.

Our kratom is carefully milled and handled with care to avoid human contact. Super Speciosa is also highly transparent with its third-party lab tests and the alkaloid content in each batch. All our products come with a scannable QR code for the accompanying lab results and a 30-day money-back guarantee. Our customer support is available for full satisfaction guarantee in case of any concerns.
“Great quality. Will buy more, surprised by the customer service. Very welcoming.”
“Quality product with quick delivery. Very reliable. Super Speciosa is the best quality I have been able to find at a reasonable price.”
Super Speciosa is a premium kratom vendor offering the highest quality kratom in the market, such as Red Maeng Da, Green Maeng Da Kratom, White Maeng Da, Red Bali Kratom, Green Bali Kratom, Green Malay, White Thai Kratom, and Red Borneo Kratom. These strains of kratom are available in various types of kratom products:

Get same-day shipping for orders placed before 2 PM Eastern.
The desired effects of kratom can be felt within 15 minutes of consumption and take about 45 minutes to completely kick in. Users have reported that kratom’s effects range from mood-lifting and increased energy levels, to relaxing and soothing, depending on the variety of strains and serving sizes.
Super Speciosa recommends all its kratom users consume no more than 2.4 grams of kratom powder per serving. This roughly equals 1 teaspoon and should not be taken more than twice in 24 hours. 5 kratom capsules and 8 tablets also equal one serving size.
You can also calculate the exact mitragynine percentage to adjust your serving size with each new batch varying in its alkaloid content. Beginners are recommended to start small.
Super Speciosa urges everyone to use kratom safely as it has not yet been approved by the U.S. Food and Drug Administration (FDA) as a dietary supplement.
Heavy use of kratom may lead to side effects such as nausea, drowsiness, constipation, itchiness, headache, frequent urination, or weight loss.
Kratom supplements are not intended to help with opioid withdrawal symptoms, opioid addiction, or chronic pain relief in which case FDA-approved therapies for mental health or addiction treatment should be pursued.
What is Kratom?
Kratom is gaining popularity as a dietary supplement among natural wellness enthusiasts because of its unique range of benefits. Kratom supplements are known for energizing and relaxing effects depending on the chosen strains and serving size.
What are the origins and traditional uses of kratom?
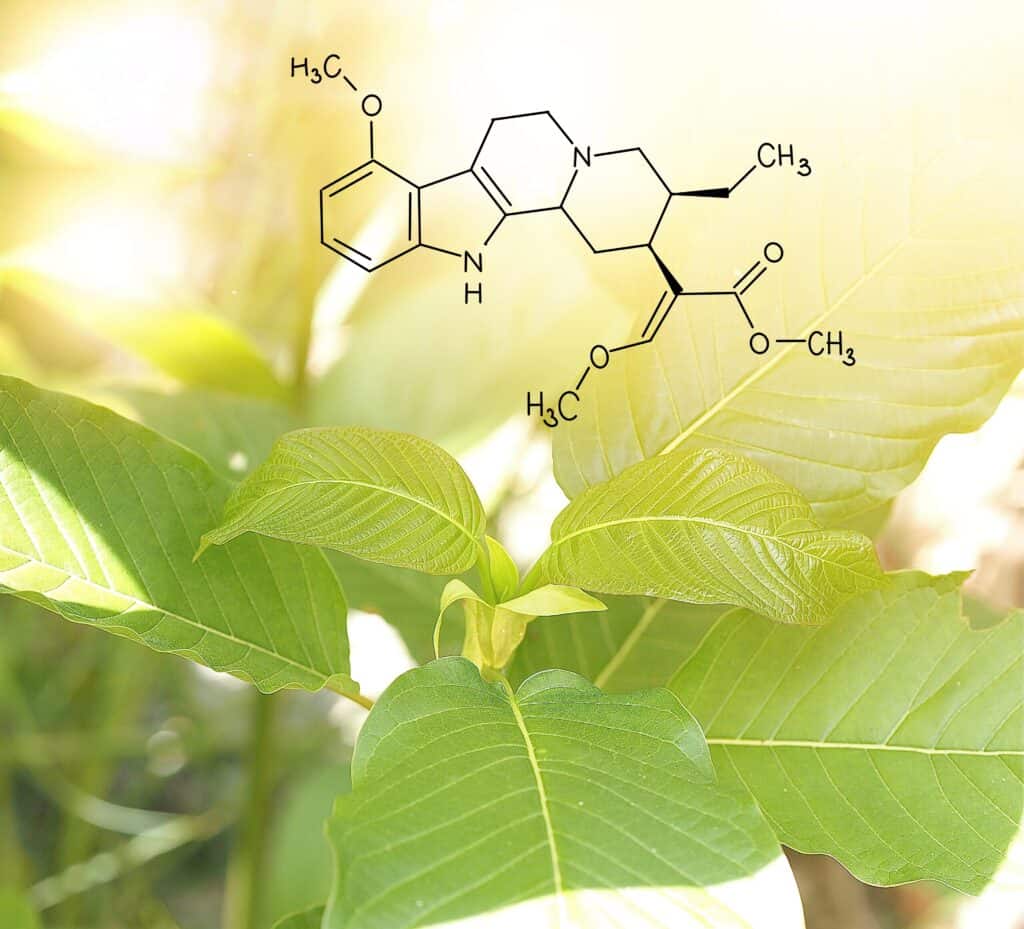
Kratom, or Mitragyna Speciosa, is a tropical tree belonging to the coffee plant family. It is typically found in the rainforests of Southeast Asia. Locals in Malaysia, Indonesia, and Thailand have been using kratom leaves for centuries to increase vitality and agility in tough working conditions.
Is it legal to buy kratom online?
Yes, it is legal to buy kratom online except in areas where kratom is illegal as no online vendor will ship if kratom is not legal in your area.
What should I look for in a kratom vendor?
A credible kratom vendor is AKA GMP-certified, is well-reputed with customer reviews, and is transparent about its kratom manufacturing processes and lab testing.
Can I buy Kratom in regular health stores or supermarkets?
Yes, you can buy kratom in regular health stores or supermarkets but it is important to consider if the available high-quality kratom products are adequately labeled and have AKA GMP-approved seals.
Are there specific brands of Kratom that are recommended?
Although there are many different kratom brands such as Kats Botanicals, Golden Monk, Kratom Spot, and Happy Go Leafy, Super Speciosa is one of the best kratom vendors to purchase kratom online. We’ve helped over 100,000 customers and have thousands of positive reviews.
How can I verify the quality of the Kratom I buy?
Look for lab results of the exact batch of kratom you are buying and also any customer reviews that offer details about their experience with the product.
Can I grow my own kratom plant for personal use?
While possible, it is extremely difficult to grow your own kratom plant as the kratom tree requires exceptional conditions and care including other risks of contamination.
What are the benefits of buying Kratom online vs. in-store?
Buying kratom products online offers a convenient shopping experience, home delivery, customer reviews, and ease of verifying the credibility of the kratom vendor.
Is it safe to buy Kratom from overseas?
Kratom from overseas if directly sourced from the supplier can be potentially dangerous as it is packaged poorly and often fails microbial testing for safe consumption.
What kratom strain should I buy for sleep?
Red vein kratom strains such as Red Maeng Da Kratom and Red Borneo Kratom are generally known for their calming effects suited for soothing sleep. Green vein kratom and white vein kratom are known for their stimulating benefits of kratom.









These statements and products presented on this website have not been evaluated by the Food and Drug Administration FDA. The products mentioned on this website are not intended to diagnose, prevent, treat or cure any diseases or health conditions. Therefore any information on this website is presented solely as the opinions of their respective authors who in which do not claim in any way shape or form to be medical professionals providing medical advice. SuperSpeciosa.com and its owners or employees cannot be held responsible for, and will not be liable for the inaccuracy or application of any information whatsoever herein provided. By purchasing our products you agree that you are aware and in compliance with your local county, state, or federal regulations. Must be 21 years or older to purchase Kratom. The US FDA has not approved kratom as a dietary supplement. We do not ship to the following states, cities and counties in the US where Kratom is banned: Alabama, Arkansas, Indiana, Rhode Island, Vermont, Wisconsin, Sarasota County, FL, Union County, MS, San Diego, CA, Jerseyville, IL, Oceanside, CA, and Ontario, OR. Furthermore, Kratom is also banned in the following countries where shipment cannot be executed: Australia, Burma, Denmark, Finland, Israel, Lithuania, Malaysia, Myanmar, Poland, Romania, South Korea, Sweden, Thailand, United Kingdom, Vietnam.
This product should be used only as directed on the label. It should not be used if you are pregnant or nursing. Consult with a physician before use if you have a serious medical condition or use prescription medications. A Doctor’s advice should be sought before using this and any supplemental dietary product. All trademarks and copyrights are property of their respective owners and are not affiliated with nor do they endorse this product. By using this site, you agree to follow the Privacy Policy and all Terms & Conditions printed on this site. Void where prohibited by law.
† These statements have not been evaluated by the FDA. This product is not intended to diagnose, treat, cure or prevent any disease.
Testimonials on this website are not intended as claims that our products can be used to diagnose, treat, cure, mitigate or prevent any disease. Read full disclaimer.


By submitting you agree to receive automated promotional messages. This agreement is not a condition of any purchase. See Terms and Privacy Policy. Frequency varies. Can opt out at any time.

There’s always something new happening. Enter your info below to get updates on huge deals, new products and special batch kratom releases.
By submitting you agree to receive automated promotional messages. This agreement is not a condition of any purchase. Message and data rates may apply. See Terms and Privacy Policy. Frequency varies. Can opt out at any time.