
A lack of regulatory standards and federal guidance on kratom’s legality has created a patchwork of laws, production standards, and consumer options.
Now, that gray area is at the heart of a lawsuit filed by a Florida woman’s family in the wake of her death.
The family of Krystal Talavera filed a lawsuit last week in federal court against Grow LLC, a kratom distributor, after Talavera was found dead in her home. At the heart of the lawsuit are a number of citations and references to guidance from the federal government–the issue though, is that many of those footnotes actually contradict available studies and statements from the National Institute on Drug Abuse.
That leaves Talavera’s family, along with other kratom consumers and customers, experiencing the turbulence and risk that comes with the current ‘hands-off’ approach by the federal government.
Talavera was a hospice nurse, who was 39 when she was found unresponsive in her living room in June of 2021. Nearby was an open container of a kratom product called “Space Dust”, the lawsuit alleges, and her death was ruled to be caused by “acute mitragynine intoxication” per the filing. No medical report or toxicology details are included in the lawsuit, nor are there any medical or scientific studies included in the footnotes.
This is where the guidance from federal agencies comes into the picture. Using a variety of publications from the Food and Drug Administration, as well as the Drug Enforcement Administration’s fact sheet on kratom, the lawsuit attempts to paint the picture of a substance that is well known to carry a risk of death. If you follow the links and look at the primary sources, however, there are no scientific or medical claims that match the wording of the lawsuit.
One of the documents cited is an FDA warning on illegally labeled kratom products that are not approved for medical purposes. While the lawsuit specifically mentions “abuse, dependence, addiction, overdose, and death” as serious health risks, the FDA warning pertains to using products with unsubstantiated claims. Per the warning, “using products with unsubstantiated claims may prevent those addicted to opioids from seeking treatments” and thus reliance on those products “may delay their path to recovery and put them at greater risk of addiction, overdose and death.”
The DEA fact sheet does not list death or overdose as a possible effect or an impact on the body.
So while kratom is not legally allowed to be marketed as a treatment option for specific uses, the documents cited short of claiming that kratom use alone causes those serious health risks. Even the document cited on the next page, a statement from the FDA commissioner, doesn’t go as far as to say that kratom outright can cause death. The statement mentions 36 deaths associated with kratom products, like in the lawsuit, but also mentions reports of kratom being laced with other opioids. The lawsuit does not disclose the full toxicology report from the autopsy.
What is mentioned in the statement, is a call for researchers “to conduct the research that will help us better understand kratom’s risk and benefit profile.” That statement, cited in last week’s lawsuit, is from April of 2018. In the four and a half years since, research has started to emerge about that risk and benefit profile.
In an article published in March of this year, researchers used a number of studies and data sets to dispel specific concerns around the use of kratom. That paper specifically addresses how media reporting and “sensationalized” cases can have adverse effects on the discussion around kratom, both for consumers and lawmakers, but it also goes a step further on the subject of overdose potential.
Both the lawsuit filed by the Talavera family and the FDA warnings allude to kratom affecting the human body the same ways as classical opioids. According to the paper from March, however, studies in animals suggest one key difference.
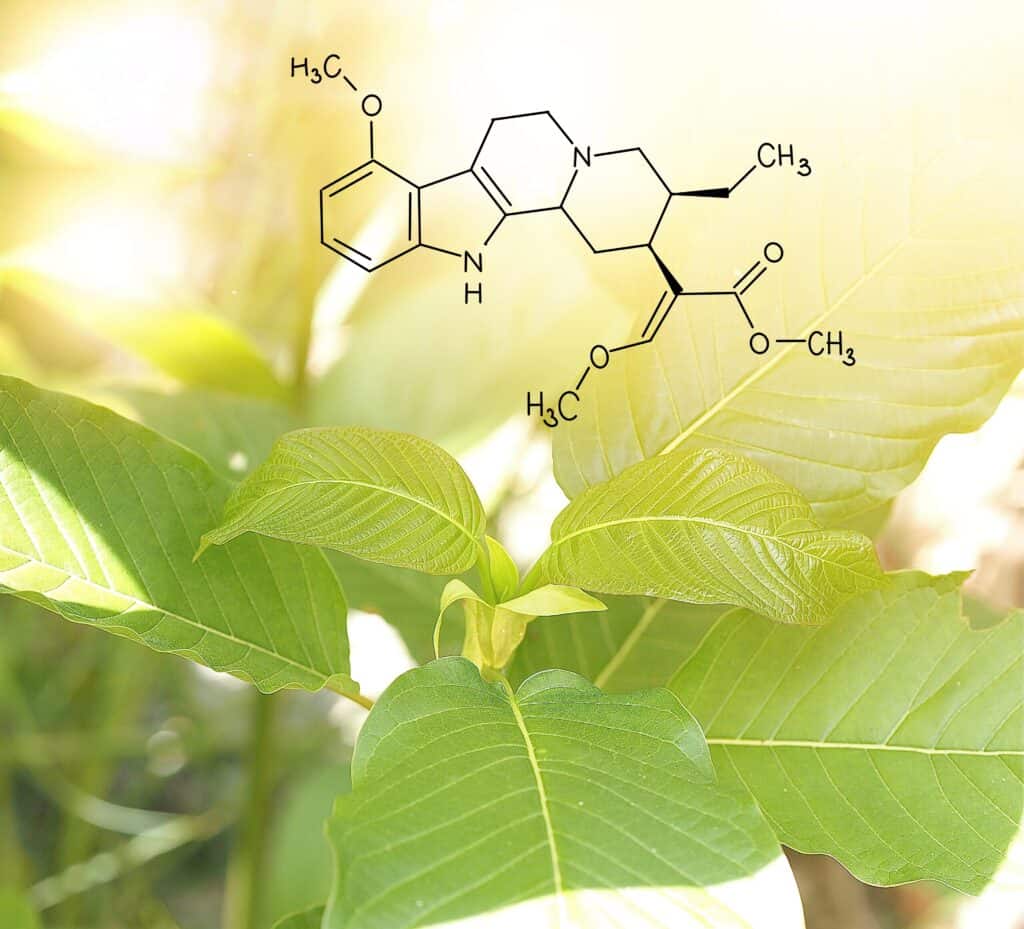
“In vitro studies reveal that biochemical pathways responsible for the analgesic and sedating effects of kratom do not carry risk of overdose comparable to classical opioids,” reads the report, which was published in the National Library of Medicine. “It is likewise important to keep in mind that while many effects of kratom are mediated by opioid receptors, kratom’s pharmacology indicates additional non-opioid mechanisms of action, including for mitragynine, again underscoring the complexity of the plant and our limited knowledge of its pharmacology.”
The focus of the lawsuit that is backed up by federal guidelines is the language that addresses a potential adulteration of the substance, and questions around the production process of the distributor.
Photos of the products used by Talavera show unlabeled pouches of powder, with letter designations drawn by hand on the pouches as the only form of identification of the substance inside. For many, this is the area where advocates have pushed for government oversight. In places like Colorado, where state law sets forth standards for regulating the kratom industry, consumers are protected by guidelines that set age limits and call for clear identification of ingredients and where the product came from.
That law is backed by NIDA research and guidance, whose kratom fact page calls fatal overdose from kratom alone “extremely rare” and instead points out how harmful contaminants or certain drug interactions can increase the risk of the substance.
“Compared to deaths from other drugs, a very small number of deaths have been linked to kratom products and nearly all cases involved other drugs or contaminants.”









These statements and products presented on this website have not been evaluated by the Food and Drug Administration FDA. The products mentioned on this website are not intended to diagnose, prevent, treat or cure any diseases or health conditions. Therefore any information on this website is presented solely as the opinions of their respective authors who in which do not claim in any way shape or form to be medical professionals providing medical advice. SuperSpeciosa.com and its owners or employees cannot be held responsible for, and will not be liable for the inaccuracy or application of any information whatsoever herein provided. By purchasing our products you agree that you are aware and in compliance with your local county, state, or federal regulations. Must be 21 years or older to purchase Kratom. The US FDA has not approved kratom as a dietary supplement. We do not ship to the following states, cities and counties in the US where Kratom is banned: Alabama, Arkansas, Indiana, Rhode Island, Vermont, Wisconsin, Sarasota County, FL, Union County, MS, San Diego, CA, Jerseyville, IL, Oceanside, CA, and Ontario, OR. Furthermore, Kratom is also banned in the following countries where shipment cannot be executed: Australia, Burma, Denmark, Finland, Israel, Lithuania, Malaysia, Myanmar, Poland, Romania, South Korea, Sweden, Thailand, United Kingdom, Vietnam.
This product should be used only as directed on the label. It should not be used if you are pregnant or nursing. Consult with a physician before use if you have a serious medical condition or use prescription medications. A Doctor’s advice should be sought before using this and any supplemental dietary product. All trademarks and copyrights are property of their respective owners and are not affiliated with nor do they endorse this product. By using this site, you agree to follow the Privacy Policy and all Terms & Conditions printed on this site. Void where prohibited by law.
† These statements have not been evaluated by the FDA. This product is not intended to diagnose, treat, cure or prevent any disease.
Testimonials on this website are not intended as claims that our products can be used to diagnose, treat, cure, mitigate or prevent any disease. Read full disclaimer.


By submitting you agree to receive automated promotional messages. This agreement is not a condition of any purchase. See Terms and Privacy Policy. Frequency varies. Can opt out at any time.

There’s always something new happening. Enter your info below to get updates on huge deals, new products and special batch kratom releases.
By submitting you agree to receive automated promotional messages. This agreement is not a condition of any purchase. Message and data rates may apply. See Terms and Privacy Policy. Frequency varies. Can opt out at any time.