
Due to a lack of trials and extensive research on the effects of kratom, the debate over what to do in the meantime is picking up steam.
Now, consumers face the potential of losing access to kratom as lingering fears over kratom’s safety permeate down from federal agencies that have been waiting years for more information and research to work its way through the pipeline.
Advocates feel there is a more even-handed approach: Testing, regulation, and making sure vendors and producers alike take certain steps to reduce safety risks.
In terms of substances monitored or controlled by the government, kratom holds a special place as a substance that exists in a legal gray area. Attempts to schedule kratom were abandoned in the face of resistance, which means there are no federal laws against kratom sales and distribution. At the same time, the same regulatory agencies that made those attempts have held firm in their belief that kratom is a substance best avoided until thorough testing can be completed.
The only problem with that line of reasoning is that it doesn’t hold up to prevailing scientific information.
And that mismatch of facts is at the center of the back-and-forth between advocates and local governments about whether to ban kratom outright, or take steps to ensure that consumers have access to an unadulterated version of the herb.
Across the country, local municipalities and states are increasingly having discussions about kratom that mirror that “big picture back-and-forth” between science and the perception of kratom as a high-risk substance. In Arkansas, where a state representative is trying to revive a failed attempt to ban kratom, voices from both sides almost perfectly mirrored the debate being had by the wider scientific/regulatory community.
On one side was a local physician, testifying that he had not seen sufficient data to show a medical use for the substance, and even going as far to say the risk outweighs any perceived benefit. From a technical standpoint, his perspective is correct: Kratom currently does not have any prescribed or accepted medical uses.
The issue, rather, came from the assertion of a perceived risk from kratom. A senior fellow from the American Kratom Association also testified for lawmakers, and made his case for a system that regulates and monitors kratom sales. Mac Haddow disagreed with the notion that kratom on its own presents a significant risk, and said that overdoses and other serious reactions usually only occur when the substance has been tainted with other products.
A similar debate is playing out among the United States’ top regulatory and medical agencies.
On the side of regulation, lab testing and further research stands the National Institute on Drug Abuse, whose page on kratom directly addresses the concern “that kratom products may cause serious harm.” Although the NIDA is quick to remind consumers that kratom still has no accepted medical use, the talking points on kratom safety seem to favor a regulatory model.
First on its list of kratom safety concerns is an echo of Haddow’s standpoint, that one of the most prominent risks associated with kratom is the possibility of contaminants. Specifically mentioned is the potential presence of heavy metal contamination and harmful bacteria. Beyond that, the NIDA’s fact page warns of possible adverse reactions to other drugs and medically prescribed substances.
As far as the risk of death, the NIDA’s page states “a very small number of deaths have been linked to kratom products compared to deaths from other drugs.” By their count, 11 deaths between 2011 and 2017 were “associated with kratom exposure” and just two occurred when kratom was the only substance involved. Available research cited by the NIDA backs up this point.
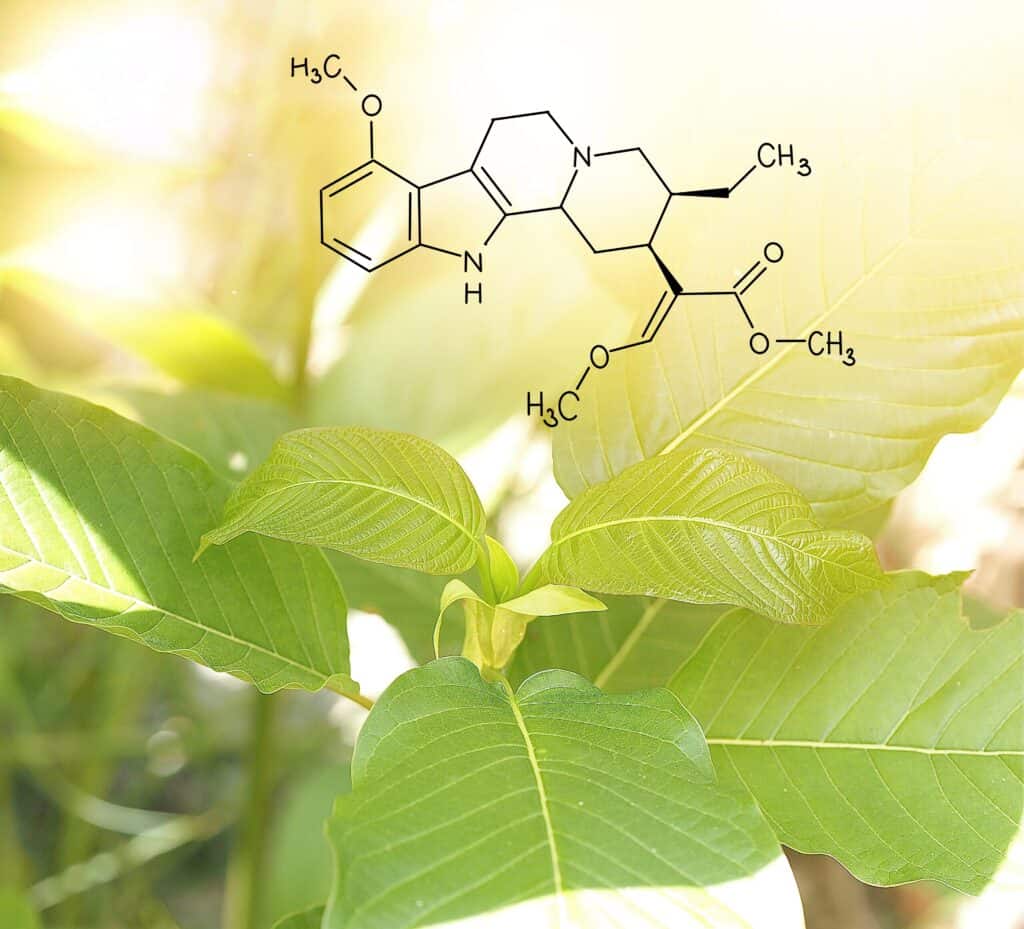
That’s why kratom advocates are pushing for regulation over prohibition, and a handful of players in the market are already taking steps to show how that could work. According to Super Speciosa’s information page on lab testing, the kratom brand is actively adapting to the concerns listed by the NIDA.
In accordance with the American Kratom Association, the group represented by Haddow, Super Speciosa adapted the Kratom GMP Standards Program more than three years ago, and tests each batch of raw product it receives as part of the preparation process.
To take it a step further, Super Speciosa also endorses other methods that would fit into a larger, Colorado-style approach to regulation, licensing and information for vendors and consumers to assure a safe, lab-tested product. In addition to the AKA standards program, Super Speciosa also adheres to criteria from the National Sanitation Foundation for dietary supplements. The lab-testing information page also advocates for labeling of batches and lots, which is similar to how legal cannabis is regulated in the United States.









These statements and products presented on this website have not been evaluated by the Food and Drug Administration FDA. The products mentioned on this website are not intended to diagnose, prevent, treat or cure any diseases or health conditions. Therefore any information on this website is presented solely as the opinions of their respective authors who in which do not claim in any way shape or form to be medical professionals providing medical advice. SuperSpeciosa.com and its owners or employees cannot be held responsible for, and will not be liable for the inaccuracy or application of any information whatsoever herein provided. By purchasing our products you agree that you are aware and in compliance with your local county, state, or federal regulations. Must be 21 years or older to purchase Kratom. The US FDA has not approved kratom as a dietary supplement. We do not ship to the following states, cities and counties in the US where Kratom is banned: Alabama, Arkansas, Indiana, Rhode Island, Vermont, Wisconsin, Sarasota County, FL, Union County, MS, San Diego, CA, Jerseyville, IL, Oceanside, CA, and Ontario, OR. Furthermore, Kratom is also banned in the following countries where shipment cannot be executed: Australia, Burma, Denmark, Finland, Israel, Lithuania, Malaysia, Myanmar, Poland, Romania, South Korea, Sweden, Thailand, United Kingdom, Vietnam.
This product should be used only as directed on the label. It should not be used if you are pregnant or nursing. Consult with a physician before use if you have a serious medical condition or use prescription medications. A Doctor’s advice should be sought before using this and any supplemental dietary product. All trademarks and copyrights are property of their respective owners and are not affiliated with nor do they endorse this product. By using this site, you agree to follow the Privacy Policy and all Terms & Conditions printed on this site. Void where prohibited by law.
† These statements have not been evaluated by the FDA. This product is not intended to diagnose, treat, cure or prevent any disease.
Testimonials on this website are not intended as claims that our products can be used to diagnose, treat, cure, mitigate or prevent any disease. Read full disclaimer.


By submitting you agree to receive automated promotional messages. This agreement is not a condition of any purchase. See Terms and Privacy Policy. Frequency varies. Can opt out at any time.

There’s always something new happening. Enter your info below to get updates on huge deals, new products and special batch kratom releases.
By submitting you agree to receive automated promotional messages. This agreement is not a condition of any purchase. Message and data rates may apply. See Terms and Privacy Policy. Frequency varies. Can opt out at any time.